
With only these three examples it indeed appears that speed is correlated to density, yet including only a few more examples would show this assumption to be incorrect. This is usually illustrated by presenting data for three materials, such as air, water and steel. Some textbooks mistakenly state that the speed of sound increases with increasing density. In general, solids will have a higher speed of sound than liquids, and liquids will have a higher speed of sound than gases. At the same time, sound will travel faster in iron than hydrogen, because the internal bonds in a solid like iron are much stronger than the gaseous bonds between hydrogen molecules. For instance, sound will travel faster in iron than uranium, and faster in hydrogen than nitrogen, due to the lower density of the first material of each set. All other things being equal, sound will travel more slowly in denser materials, and faster in stiffer ones. In a real material, the stiffness of the springs is called the elastic modulus, and the mass corresponds to the density. Effects like dispersion and reflection can also be understood using this model. The speed of sound through the model depends on the stiffness of the springs (stiffer springs transmit energy more quickly). Sound passes through the model by compressing and expanding the springs, transmitting energy to neighboring balls, which transmit energy to their springs, and so on. For a real material the balls represent molecules and the springs represent the bonds between them. The transmission of sound can be explained using a toy model consisting of an array of balls interconnected by springs. A derivation of these equations will be given in a later section.
It gives good predictions in relatively dry, cold, low pressure conditions, such as the Earth's stratosphere.
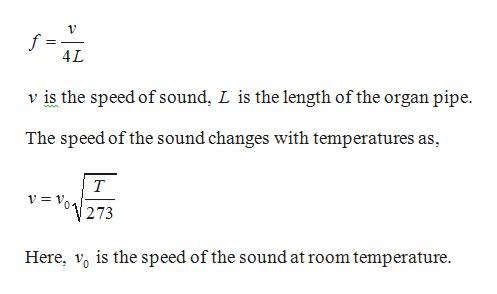
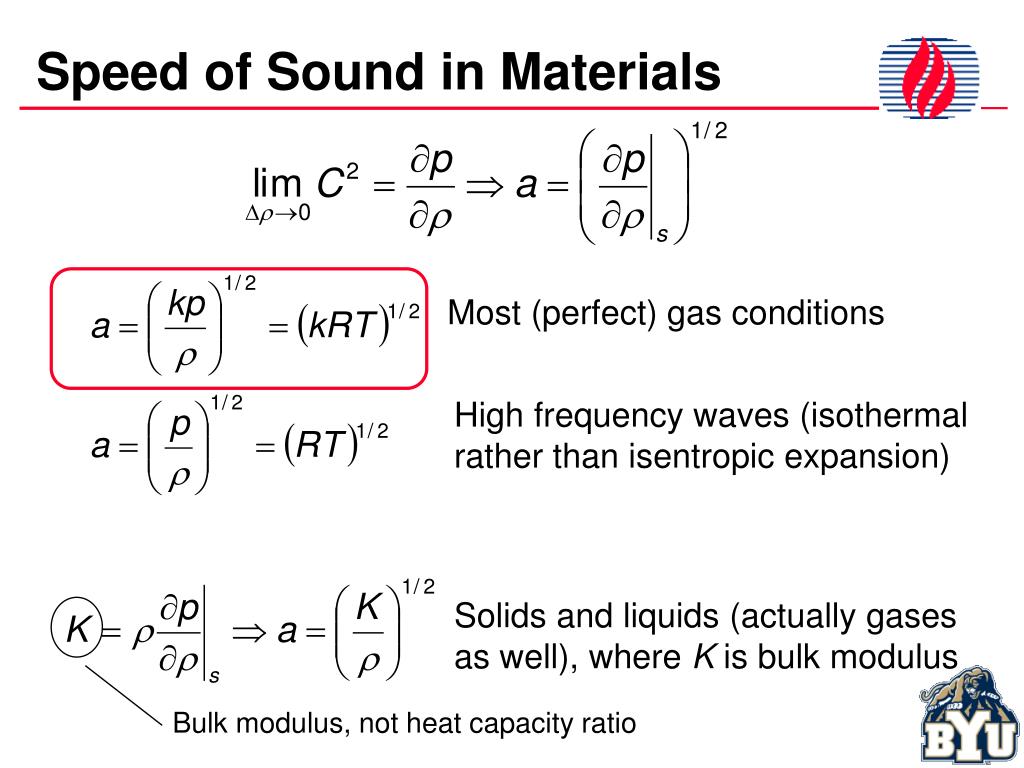
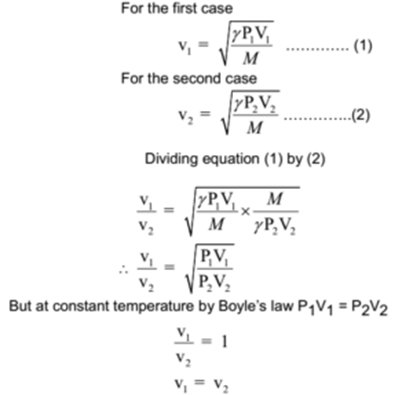
This equation is correct to a much wider temperature range, but still depends on the approximation of heat capacity ratio being independent of temperature, and will fail, particularly at higher temperatures. If ideal gas γ is assumed to be 7/5 = 1.4 exactly, the 0 ☌ speed is calculated (see section below) to be 331.3 m/s, the coefficient used above. Commonly found values for the speed of sound at 0 ☌ may vary from 331.2 to 331.6 due to the assumptions made when it is calculated. The value of 331.3 m/s, which represents the 0 ☌ speed, is based on theoretical (and some measured) values of the heat capacity ratio, γ, as well as on the fact that at 1 atm real air is very well described by the ideal gas approximation. This equation is derived from the first two terms of the Taylor expansion of the following much more accurate equation: Where is the temperature in degrees Celsius (☌). s -1), at temperatures near 0 ☌, can be calculated from:.The approximate speed of sound in dry (0% humidity) air, in metres per second ( m However, in the stratosphere, the speed of sound increases with height due to heating within the ozone layer, producing a positive sound speed gradient. The decrease of the sound speed with height is referred to as a negative sound speed gradient. Since temperature and thus the speed of sound normally decrease with increasing altitude, sound is refracted upward, away from listeners on the ground, creating an acoustic shadow at some distance from the source. In the Earth's atmosphere, the most important factor affecting the speed of sound is the temperature (see Details below). Humidity also has a small, but measurable effect on sound speed (increase of about 0.1%-0.6%), because some oxygen and nitrogen molecules of the air are replaced by the lighter molecules of water. In non-ideal gases, such van der Waals gas, the proportionality is not exact, and there is a slight dependence on the gas pressure even at a constant temperature. At a constant temperature, the ideal gas pressure has no effect on the speed of sound, because pressure and density (also proportional to pressure) have equal but opposite effects on the speed of sound, and the two contributions cancel out exactly. In a given ideal gas the sound speed depends only on its temperature. For example, in low molecular weight gases, such as helium, sound propagates faster compared to heavier gases, such as xenon.
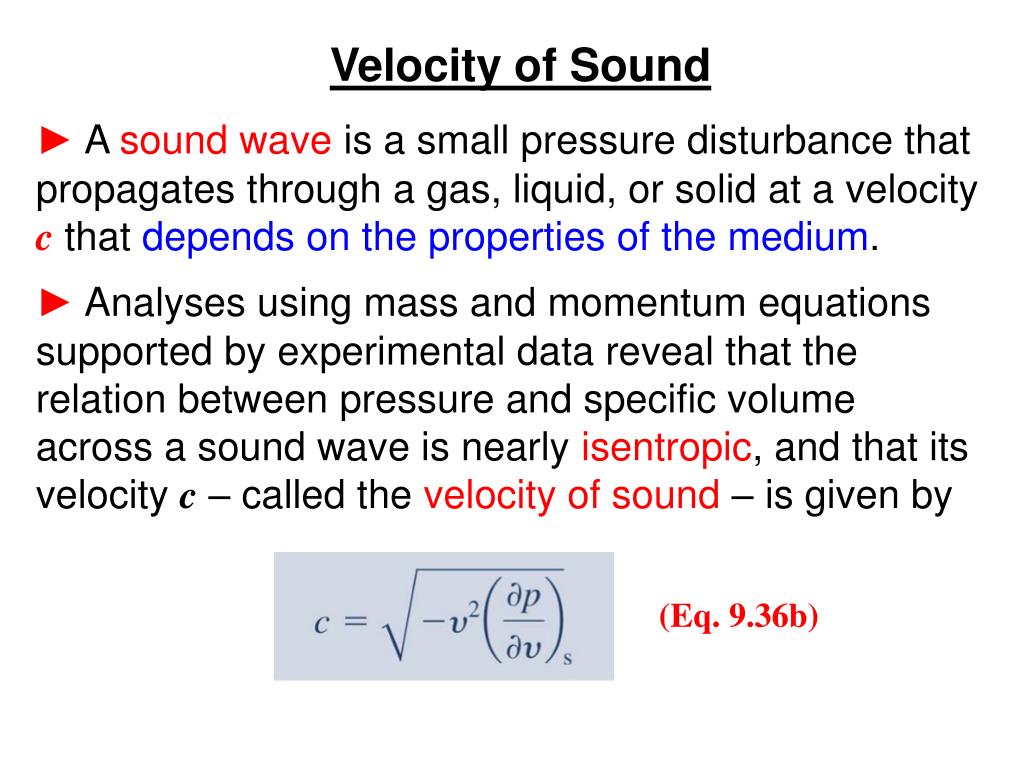
The speed of sound is variable and depends mainly on the temperature and the properties of the substance through which the wave is traveling.


 0 kommentar(er)
0 kommentar(er)
